Connector Degradation Mechanisms—Corrosion Part II
Connector Basics: Connector Degradation Mechanisms—Corrosion Part II
This is the third article in a series on connector degradation mechanisms and the second article focused on corrosion. Tin-to-tin contact interfaces and fretting corrosion, as the dominant degradation mechanism in such systems, was the focus of the previous article. Now, we will address corrosion effects on contact resistance in noble metal contact systems, particularly gold-plated interfaces. The key point in this article is the importance of a nickel underplate beneath the gold and its benefits in enhancing the performance of gold-plated connectors with respect to corrosion degradation. The following paragraph is repeated from the previous article for convenience, because it provides necessary background to understand the kinetics of corrosion-related degradation.
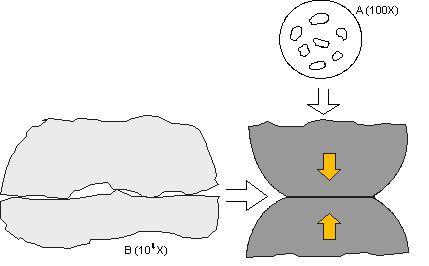
In the first article, the asperity, or a-spot, model of a contact interface was introduced. Figures 1 and 2 of the first article are shown for convenience in the following discussion. The key point of the asperity model is that the asperity contact points are very small, on the order of microns in diameter, and are distributed across an apparent contact area determined by the geometry of the contact springs at the interface, and the contact force exerted by the springs due to their deflection on mating. The electrical current across the contact interface must flow through the asperity contact points, resulting in a resistance called constriction resistance. The magnitude of the constriction resistance depends on the number, size, and distribution of the asperity contacts at the interface, because all the asperity contacts are electrically in parallel. Constriction resistance exists even in the ideal case when all the asperity contact interfaces are metal-to-metal, e.g. gold-to-gold or tin-to-tin. If any of the asperity interfaces are compromised by corrosion films or contaminants, the constriction resistance will increase. This is the reason why corrosion is a degradation mechanism for connectors. Loss of asperity contact area, or of asperity contacts, due to corrosion or contamination, can result in contact interface resistance increases sufficient to lead to connector failures.
 Now, let’s move onto corrosion degradation in gold-plated connectors. It is well known that gold is a noble metal. That is, a metal that does not corrode, a characteristic that accounts for the widespread use of gold in jewelry. While that is a true statement, it does not mean that gold-plated connectors are not susceptible to corrosion. Connectors are an electromechanical system. They perform an electrical function, to provide electrical continuity between two subsystems through a mechanical spring system, which provides the desired mating and contact force characteristics. In the case of gold-plated connectors, the system consists of the gold surface plating and a nickel underplate (to optimize the contacting surface) over a copper alloy spring material. The copper alloy spring material provides the necessary spring characteristics on connector mating. The corrosion source in this system is the copper alloy spring material. Copper reacts with oxygen, sulfur, and chlorine, common components of application environments where connectors are used. So, with respect to corrosion susceptibility, connector design must address how to eliminate, or reduce copper corrosion, and ensure that any corrosion products that do form cannot reach the connector contact interface. This is easy in principle, but a challenge in practice, as we shall see.
Now, let’s move onto corrosion degradation in gold-plated connectors. It is well known that gold is a noble metal. That is, a metal that does not corrode, a characteristic that accounts for the widespread use of gold in jewelry. While that is a true statement, it does not mean that gold-plated connectors are not susceptible to corrosion. Connectors are an electromechanical system. They perform an electrical function, to provide electrical continuity between two subsystems through a mechanical spring system, which provides the desired mating and contact force characteristics. In the case of gold-plated connectors, the system consists of the gold surface plating and a nickel underplate (to optimize the contacting surface) over a copper alloy spring material. The copper alloy spring material provides the necessary spring characteristics on connector mating. The corrosion source in this system is the copper alloy spring material. Copper reacts with oxygen, sulfur, and chlorine, common components of application environments where connectors are used. So, with respect to corrosion susceptibility, connector design must address how to eliminate, or reduce copper corrosion, and ensure that any corrosion products that do form cannot reach the connector contact interface. This is easy in principle, but a challenge in practice, as we shall see.
If connector manufacturers had the luxury of completely plating the contact springs with, say, five microns of gold, corrosion would not be an issue in connectors. Cost effectiveness, however, dictates much thinner platings—0.25 to 0.75 microns are typical, with the platings being applied only at the contact interface itself, selective plating. Meeting this economic requirement introduces two potential sources of exposed copper spring material. Selective plating, as well as other manufacturing processes, may lead to bare copper and bare copper edges. Thin platings lead to potential copper exposure where defects exist in the gold plating. In addition to these intrinsic corrosion sources, wear of the gold plating during the mating cycles of the connector, or disturbances of the contact interface during application, must be taken into consideration. All of these issues are addressed by an often overlooked and underappreciated component of the gold connector system, the nickel underplate.
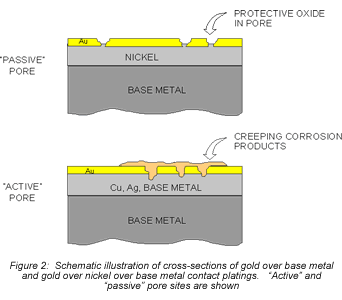 Consider first the intrinsic issues, starting with plating thickness. Figure 2 schematically illustrates gold-plated surfaces with and without a nickel underplate. Any defect in the gold-only plating will result in exposure of the copper alloy spring material. Plating defects include porosity, scratches, and incomplete surface coverage due to contaminants on the base metal prior to plating, among others. The potential for all of these defects is increased as plating thickness decreases. Given the presence of such plating defects, any exposed copper alloy will react with the application environment and the corrosion products formed can make their way to the surface of the plating. For the purposes of this discussion, I will simply note that copper-sulfur corrosion products are known to migrate, or creep, over metal surfaces as indicated in Figures 2 and 3. Figure 2 schematically indicates the migration of corrosion products up the walls of the pore site to the surface. Figure 3 is a photomicrograph of corrosion migration rings on the surface of a gold-plated copper alloy coupon. If the contact interface incorporated any of the corrosion product rings indicated in Figure 3, the interface resistance would most likely be compromised to some degree. Use of a nickel underplate, however, both inhibits corrosion and reduces corrosion creep. Nickel forms a very thin self-limiting and passivating oxide that does not migrate. In effect, the nickel passivates the base of the defect site so no corrosion products are able to migrate to the surface.
Consider first the intrinsic issues, starting with plating thickness. Figure 2 schematically illustrates gold-plated surfaces with and without a nickel underplate. Any defect in the gold-only plating will result in exposure of the copper alloy spring material. Plating defects include porosity, scratches, and incomplete surface coverage due to contaminants on the base metal prior to plating, among others. The potential for all of these defects is increased as plating thickness decreases. Given the presence of such plating defects, any exposed copper alloy will react with the application environment and the corrosion products formed can make their way to the surface of the plating. For the purposes of this discussion, I will simply note that copper-sulfur corrosion products are known to migrate, or creep, over metal surfaces as indicated in Figures 2 and 3. Figure 2 schematically indicates the migration of corrosion products up the walls of the pore site to the surface. Figure 3 is a photomicrograph of corrosion migration rings on the surface of a gold-plated copper alloy coupon. If the contact interface incorporated any of the corrosion product rings indicated in Figure 3, the interface resistance would most likely be compromised to some degree. Use of a nickel underplate, however, both inhibits corrosion and reduces corrosion creep. Nickel forms a very thin self-limiting and passivating oxide that does not migrate. In effect, the nickel passivates the base of the defect site so no corrosion products are able to migrate to the surface.
 An additional thickness-related benefit of nickel underplates is as a diffusion barrier. Copper diffuses readily through gold, and if diffused copper reaches the surface of the gold-plating, it will form corrosion films on the surface that may impede the desired metal-to-metal contact interface that the gold surface was intended to provide. The rate of diffusion of copper through the nickel underplate is much slower, and the nickel underplate is typically much thicker than the gold-plating, so there is a significant reduction in the rate of copper diffusion to the gold surface. Because diffusion rates increase with temperature, this nickel benefit is, of course, much more important if the connector is intended for higher temperature applications.
An additional thickness-related benefit of nickel underplates is as a diffusion barrier. Copper diffuses readily through gold, and if diffused copper reaches the surface of the gold-plating, it will form corrosion films on the surface that may impede the desired metal-to-metal contact interface that the gold surface was intended to provide. The rate of diffusion of copper through the nickel underplate is much slower, and the nickel underplate is typically much thicker than the gold-plating, so there is a significant reduction in the rate of copper diffusion to the gold surface. Because diffusion rates increase with temperature, this nickel benefit is, of course, much more important if the connector is intended for higher temperature applications.
The passivating and migration inhibiting characteristics of nickel are also beneficial with respect to selective plating. If the copper alloy spring material is plated overall with nickel, prior to selective gold-plating of the contact interface, surface and edge corrosion (and the associated corrosion creep to the contact interface) will be minimized.
In addition to these intrinsic issues, the potential corrosion effects of wear on the plating are also moderated by characteristics of the nickel underplate. As mentioned, wear-through can occur due to the mating cycles of the connector or through micromotions of the contact interface due to mechanical or thermal expansion driving forces. There are two nickel benefits to minimize wear-through as a potential degradation mechanism. The first is the passivation and migration inhibition characteristic of nickel as previously discussed. Wear-through of the gold and exposure of the nickel underplate does not lead to corrosion-related degradation. It is possible, however, that the exposed nickel may not be as effective a metal-to-metal contact interface as gold-to-gold, so an increased contact resistance may result. The magnitude of such an increase would be far less than what would occur due to corrosion effects. The second benefit provided by a nickel underplate with respect to wear-through is an improvement in the wear resistance of the contact plating. Wear behavior will be discussed in more detail in the next installment of this series, so for now, it is sufficient to note that the nickel underplate will increase the effective hardness of the contact plating. The gold platings used on connectors are typically so-called hard golds and have Knoop hardness of the order of 200. Nickel underplate hardnesses are generally 400 Knoop or higher. Thus, the effective hardness of the plating is increased and wear rates tend to decrease as the surface hardness increases.
Given the importance of the benefits of a nickel underplate to connector performance, what nickel thickness is necessary? A typical range of nickel thickness in gold-plated connectors is in the range of 1.25 to 4.0 microns. The lower limit is to ensure sufficient thickness to provide the basic benefits described. The upper limit is determined by both cost-benefit and mechanical considerations. The cost-benefit issue is obvious, more nickel means more plating time and material cost. The mechanical considerations are more complicated. As nickel plating thicknesses increase, the ductility of the nickel tends to decrease and the roughness of the plating tends to increase. Reduced ductility can lead to cracking of the plating, and increased roughness to compromised porosity and wear performance.
In summary, the importance of a nickel underplate in gold-plated connector systems cannot be overemphasized. The passivating characteristic of nickel is significant in moderating the formation and migration of corrosion products arising from any exposed copper surface on the contact spring. In addition, nickel provides a diffusion barrier against base metal constituents of the contact spring. The hardness of nickel is important in improving the wear resistance of gold-plated contact systems to improve both the mating cycle life of connectors and resistance to fretting wear. Given these benefits, a nickel underplate should always be specified for any gold-plated connector system.
- Nanocrystalline Silver Alloy Contact Finishes in Electronic Applications - April 6, 2015
- Nanocrystalline Silver Contact Platings - March 16, 2015
- Dr. Bob on Gold Flash Contact Finishes (and Max Peel) - September 22, 2014