Implantable Medical Devices: Smart Devices, Healthy Patients
Implantable Medical Devices: Smart Devices, Healthy Patients
Development of microminiature packaging and interconnection techniques is a key enabling factor in the realization of smaller, lighter, lower-power-consuming electromedical devices. Modern compact devices have higher levels of data acquisition and signal processing, so the new standards in medical devices are greater density on a smaller form factor.
Miniaturization is enabled by advances in chip-scale packaging techniques and package-free options, as well as by semiconductors etched on films and laminates. Market demand for the miniaturization of medical implants continues to increase because it facilitates less-invasive implantation procedures. In addition, smaller-profile implants are often less visible or less obtrusive under a patient’s skin, thereby increasing patient comfort and enhancing quality of life.
Implantable Medical Devices
 An implantable medical device is inserted into the human body and expected to stay there for 30 days or more. An active implantable medical device is inserted into the human body, uses electricity or other energy, and is expected to stay there after the medical/surgical procedure is completed. Implantable devices serve a variety of functions, from vascular stents that preserve blood flow, to electrostimulation devices that regulate heart rhythm or block spurious signals in the brain, to orthopedic devices that mechanically reinforce the spine or restore range of motion of hips and knees.
An implantable medical device is inserted into the human body and expected to stay there for 30 days or more. An active implantable medical device is inserted into the human body, uses electricity or other energy, and is expected to stay there after the medical/surgical procedure is completed. Implantable devices serve a variety of functions, from vascular stents that preserve blood flow, to electrostimulation devices that regulate heart rhythm or block spurious signals in the brain, to orthopedic devices that mechanically reinforce the spine or restore range of motion of hips and knees.
Implantable devices such as pacemakers and defibrillators are increasingly connected through wireless radios to networked monitoring systems that upload data to manufacturers. Patients with these smart devices can remotely upload device and physiological data to doctors, who use the information to coordinate care and track symptoms; but this added level of technology also makes the devices more vulnerable.
Worries over medical-device cybersecurity have largely focused on plugged-in equipment primarily used in hospitals that are vulnerable to viruses traveling across medical networks. Device experts say one of the most alarming prospects is that a cyberattacker might one day penetrate life-sustaining implanted devices.
Cardiac Rhythm Management (CRM) devices are used to ensure the proper functioning of the heart, including Implantable Cardioverter Defibrillators (ICDs), external defibrillators, pacemakers, and cardiac resynchronization therapy devices. Though growth in the CRM device market remained slow in the recent past owing to product recalls and adverse media publicity, with new technological advances, the outlook remains positive.
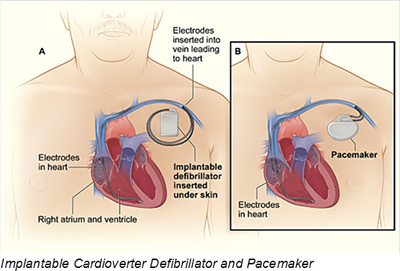 The ICD segment currently holds the largest share of the cardiac defibrillator device market. An ICD is a small device that is placed in the chest or abdomen and contains a battery, pulse generator, a small computer, and wires with electrodes on the ends that connect to the heart chambers and monitor heart rhythm. If the device detects an irregular rhythm, it will use low-energy electrical pulses to restore a normal rhythm. If the low-energy pulses do not restore normal heart rhythm, the ICD will switch to high-energy pulses for defibrillation. The ICD also can record the heart’s electrical activity and heart rhythms. Typically, batteries last for five to seven years. Most new ICDs can act as both pacemakers and defibrillators.
The ICD segment currently holds the largest share of the cardiac defibrillator device market. An ICD is a small device that is placed in the chest or abdomen and contains a battery, pulse generator, a small computer, and wires with electrodes on the ends that connect to the heart chambers and monitor heart rhythm. If the device detects an irregular rhythm, it will use low-energy electrical pulses to restore a normal rhythm. If the low-energy pulses do not restore normal heart rhythm, the ICD will switch to high-energy pulses for defibrillation. The ICD also can record the heart’s electrical activity and heart rhythms. Typically, batteries last for five to seven years. Most new ICDs can act as both pacemakers and defibrillators.
Doctors also treat arrhythmias with a pacemaker. Pacemakers have many similarities to ICD devices and can be temporary or permanent. Most pacemakers are now equipped with wireless transmission capabilities allowing patients and doctors to continuously monitor a patient’s post-operative progress. Many pacemakers monitor blood, temperature, and breathing, and can adjust your heart rate to accommodate changes in your activity. The pacemaker’s computer also records your heart’s electrical activity and heart rhythm.
Neurostimulators
Neuromodulation is a rapidly evolving medical technique that provides symptomatic relief through the alteration or modulation of nerve system function. It works either by stimulating nerves to produce a natural biological response or by applying small doses of pharmaceutical agents directly to the site of action. Neuromodulation devices are active implants that send mild electrical pulses and/or drug infusion to a target nerve to treat certain conditions.
There are two types of neurostimulators generally found in the market:
- An Implanted Pulse Generator (IPG) is fully implanted under the skin, including the battery. This type of system is best for low-to-moderate pain levels. This is because there will be little to no need to change the battery, a task that requires surgery.
- Radio Frequency (RF) systems send radio waves through an antenna to the implanted device. With RF systems, the battery remains outside the body to avoid a surgical procedure when changing or recharging the battery becomes necessary.
An IPG is a battery-powered device designed to deliver electrical stimulation to the brain. Neurostimulation devices contain sophisticated electronics packaged within a hermetically sealed titanium case. The devices apply low-level energy to the nervous system to block nerve signals between the brain and peripheral tissue or organs. Emerging areas of development include treatment of large-population diseases and disorders, including Alzheimer’s disease, chronic migraines, paralysis, sleep apnea, and stroke, with the potential to expand to other applications.
 There are three main modalities of implantable neurostimulation therapy — vagus nerve stimulation (VNS), spinal cord stimulation (SCS), and deep brain stimulation (DBS). Neurostimulators typically work by applying continuous low-level currents. The current applied for VNS and DBS ranges from 1.0 to 3.5 mA, and battery life is typically eight to 12 years. For SCS, a current of 20-25 mA is needed, requiring a larger power source and overall device volume to house a bigger battery. As the miniaturization trend for implanted devices has grown, companies developing SCS devices have opted to use rechargeable power systems. As a result, SCS systems have an internal battery that is recharged by an external power source, usually by inductive power transmission.
There are three main modalities of implantable neurostimulation therapy — vagus nerve stimulation (VNS), spinal cord stimulation (SCS), and deep brain stimulation (DBS). Neurostimulators typically work by applying continuous low-level currents. The current applied for VNS and DBS ranges from 1.0 to 3.5 mA, and battery life is typically eight to 12 years. For SCS, a current of 20-25 mA is needed, requiring a larger power source and overall device volume to house a bigger battery. As the miniaturization trend for implanted devices has grown, companies developing SCS devices have opted to use rechargeable power systems. As a result, SCS systems have an internal battery that is recharged by an external power source, usually by inductive power transmission.
The general trend in implantable devices is miniaturization. New devices tend to be smaller, but with a greater number of leads that get more signals into and out of a single device. Future neurostimulator applications may have 100 to 200 leads, which will give device manufacturers opportunities to add further treatment options to an implantable system. One of the most exciting avenues of research to increase the number of leads is the development of new high-density feedthroughs that could contain ten times the number of leads, while keeping the current size and spacing.
Another interesting development in the IPG arena is the development of “body communications,” in which ultrasonic devices are placed into a medical device casing and used to remotely power and communicate with other devices in the body. This next level of improvement could mean that no wires would have to be implanted. Implanted wires in the body may eventually fail and subsequent removal and replacement can be difficult. In addition, using ultrasound, as opposed to RF, means the communication stays within the body. This means one person’s medical device is less likely to interfere with another person’s device and it could be more readily protected against interference from MRI equipment, scanners, or other large electrical devices.
Using ultrasound to both power and interrogate remote sensors is a likely development for many implantable devices, but wiring would still be required for neurostimulators, where leads are attached to the skull, brain, or spine. However, in the future, the main implant might be able to communicate with other devices implanted in the body or the external programmer via ultrasound rather than RF. Neurostimulation devices developed within the next five years should be able to sense the onset of a neurological episode, use an algorithm to determine if therapy is appropriate and optimized, and then deliver therapy.
Prosthetics and Biomedical Electronics
As modern electronics technology merges with nanotechnology, advanced implants, and major sensor advances in medicine and biology, science fiction is becoming more of a reality. Innovation is transforming sensor-based electronics in human-body enhancements and replacements. Major advancements in the field of prosthetics have resulted from the use of microprocessor technology to operate the major joints of the prosthesis (knees, hands, and elbows). The prosthesis has a computer processor on board that reads inputs from sensors and then calculates adjustments to the prosthesis to accommodate the wearer’s activities.
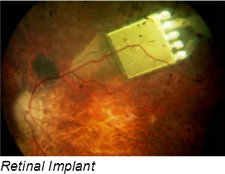 Retinal-prosthesis development can help restore sight to people assisting an eye’s lost functions with an implant that contains a 15-channel stimulator chip, discrete power-supply components, and power- and data-receiving coils that conform to the outer wall of an eye. The system has an external video-capture unit and a transmitter that wirelessly sends image data to the implanted portion of the device. A custom ASIC then translates the image into biphasic current pulses of programmable strength, duration, and frequency to the electrode array.
Retinal-prosthesis development can help restore sight to people assisting an eye’s lost functions with an implant that contains a 15-channel stimulator chip, discrete power-supply components, and power- and data-receiving coils that conform to the outer wall of an eye. The system has an external video-capture unit and a transmitter that wirelessly sends image data to the implanted portion of the device. A custom ASIC then translates the image into biphasic current pulses of programmable strength, duration, and frequency to the electrode array.
Another area of advancement in biomedical science covers cochlear implants. The primary goal of these implants is to use electrical stimulation safely to provide or restore functional hearing. A cochlear implant converts sound to electric impulses for delivery to the auditory nerve, sending impulses to the brain, which interprets the impulses as sound.
Bendable and Biodegradable Electronics

Researchers are developing bendable electronics for potential use in a range of medical device applications, from sensors that map and treat neurological disorders, to balloon catheter components that perform diagnostic and surgical functions. These applications typically benefit from compact and lightweight systems that fully conform or dynamically adapt to irregularly shaped surfaces, such as the human body. It is not specifically the chip, but rather the whole technology platform combining ultrathin bendable chips and compliant, elastic electrical interconnections, that makes the technology of interest for medical implantable devices or wearable sensing systems.
A new kind of biodegradable electronics, called “transient electronics,” is robust enough for applications such as medical implants, yet completely dissolvable in water or bodily fluids. In transient applications, the sheets are so thin that they completely dissolve in a few days when immersed in bodily fluids. Together with soluble conductors and dielectrics, based on magnesium and magnesium oxide, the technology can produce a wide range of electronic components.
Outlook
Demand for higher performance interfaces of all types will require out-of-the-box innovation using nontraditional materials and manufacturing processes. Next-generation electronic devices may require even smaller interconnects. Electronic devices at this scale will require the development of an entirely new class of connectors or may portend wireless charging as well as data I/O technology. The challenge will be for the connector industry to re-envision itself, from an adopter to a creator, of new technology capable of supporting future generations of advanced electronic products.
- State of the Industry: 2022-2023 Connector Sales - April 16, 2024
- Amphenol is On a Roll - April 2, 2024
- Nicomatic Proves That Two Heads are Better Than One - March 26, 2024

