Combating Corrosion in High-Reliability Connectors
Galvanic corrosion can be significantly attenuated through thoughtful connector design, materials selection, manufacturing, and handling practices designed to maintain high-quality contact surface integrity and minimize connector exposure to adverse conditions.
Metal contact surfaces are subject to corrosion in the form of galvanic attacks, which take place when high humidity and ambient trace chemicals combine to form an adsorbed electrolyte on the contact surface. The buildup of this insulating film on connector contact surfaces can cause corrosion, a chemical process that gradually deteriorates the surface of a metal via oxidation or chemical reactions. This is responsible for most low-voltage, low-current, signal-level circuit failures in datacom and telecom equipment ranging from tablets, laptops, and consumer-grade IoT devices like smart thermostats to Industrial IoT (IIoT) network technologies.

The occurrence of corrosion, however, also depends on the integrity and chemical activity level of the connector’s metal surface. If a contact surface must remain stable for a long period of time, which is a necessity for most datacom and telecom connectors, it can be coated with gold or other electrically conductive, corrosion-resistant precious metals using electroplating or cladding processes.
Galvanic Attack
Galvanic attack is an electrolytic process similar to the action in a battery cell. For galvanic corrosion to occur, an electrolyte, an anode, a cathode, and a current path must be present. The degree of corrosion depends on five conditions: ambient temperature, environment, humidity, the type of metal product, and the type of corrosion product.
Two environmental conditions — relative humidity in excess of 60% and the presence of ambient gases such as chlorine, nitrous oxide, and sulfur dioxide — are the chief catalysts for creating an electrolyte. High humidity levels allow water vapor that contains dissolved gasses to condense on the connector surface, creating a mildly conductive acid. Local anodes and cathodes are provided by breaches or pores in the contact surface coating. The exposed base metal at the bottom of the pore becomes the anode and the precious metal coating forms the cathode. Finally, because the coating, the interface, the base metal, and the electrolyte are all conductive, the current path is complete.
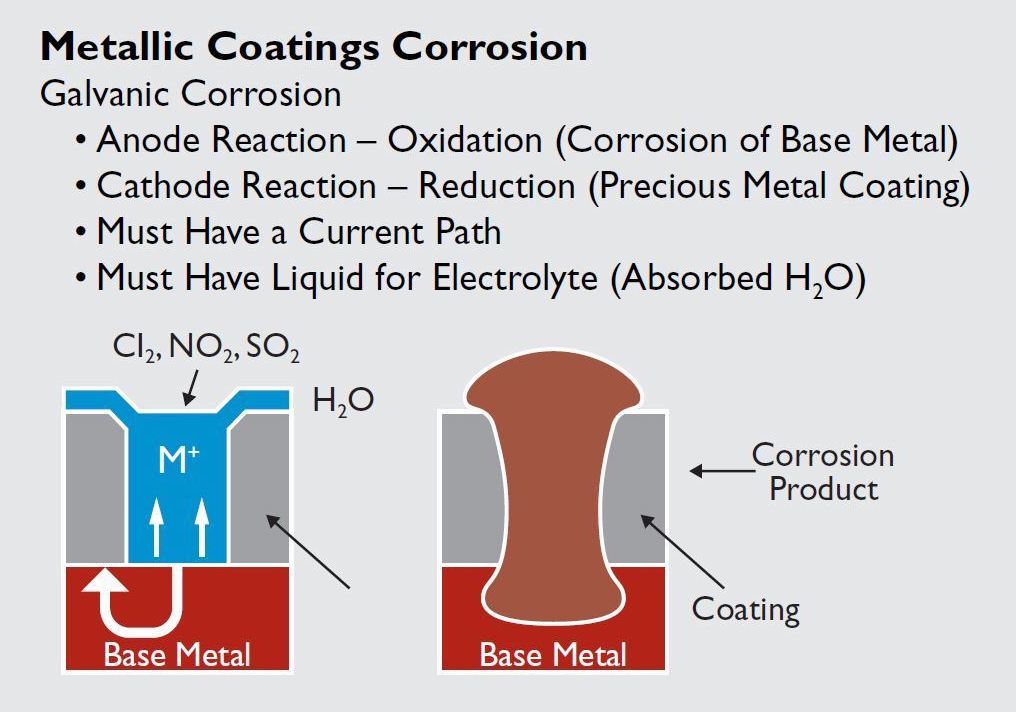
Four conditions must be present for galvanic corrosion to occur. There must be an electrolyte, an anode, a cathode, and a current path.
The rate of corrosion is a function of the corrosion product. Copper corrosion products are very porous, allowing for continuous vapor penetration, and react with water to form additional compounds that promote rapid, continuous corrosion. Products from nickel corrosion are impervious to moisture and tenaciously adhere to metal surfaces, forming a moisture barrier. As a result, galvanic attack from nickel tends to be self-limiting.
Ultimately, corrosion will occur if all four requisite conditions are present, regardless of the surface coating. To combat corrosion, at least one of the four conditions must be eliminated. Since connector manufacturers can control the quality of the contact surface and its coating, they can decrease the potential for anode and cathode formation.
Contact Surface Coating Integrity
The most common source of a breach in a contact’s surface coating is porosity, or a discontinuity in the coating that exposes the substrate. A breach can occur during the manufacture of the coating, during the coating process, or during the manufacturing, handling, and testing of a connector.
Both electroplated and clad metals are subject to excessive porosity. For electroplated metals, this condition can result from foreign particles or poor substrate surface finishes, contaminated electroplating solutions, uneven plating due to surface roughness, internal stresses that cause the coating to self-destruct in high-tension regions, and brittleness that renders the surface vulnerable to cracking during forming operations or in response to rough handling.
Clad metals, on the other hand, are more malleable and tend to maintain their integrity during forming. Porosity in clad contact surfaces is more likely to result from foreign particles on the surface of the substrate or excessive surface roughness. However, clad coatings are subject to false porosity readings caused by interdiffusion.
During the annealing portion of the cladding process, atoms from the substrate material migrate through the grain structure of the substrate and the coating material. This diffusion leaves regions of base-metal-rich alloys at grain boundary junctions on the surface of the connector material. Porosity testing will show these regions as pores, usually measuring less than 0.003″ in diameter, but this is not true porosity and should be ignored.
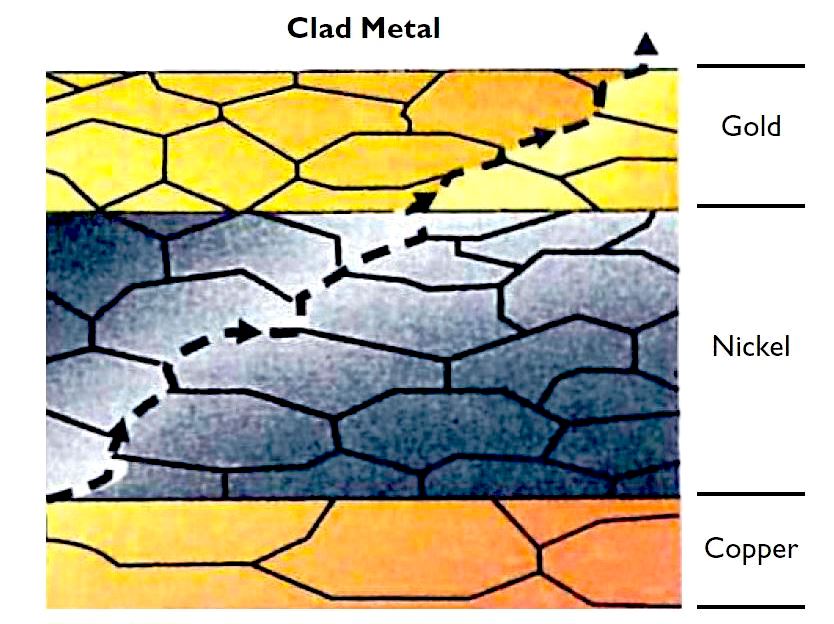
Clad contact surfaces tend to have thicker nickel barriers and longer diffusion paths than electroplated contact surfaces, which inhibits base-metal surface migration.
For plated materials, migration along grain boundaries presents an additional source of corrosion. Electroplated hard gold is about 10% organic material and these organics tend to widen the grain boundaries, giving corrosive elements an easier path to the substrate.
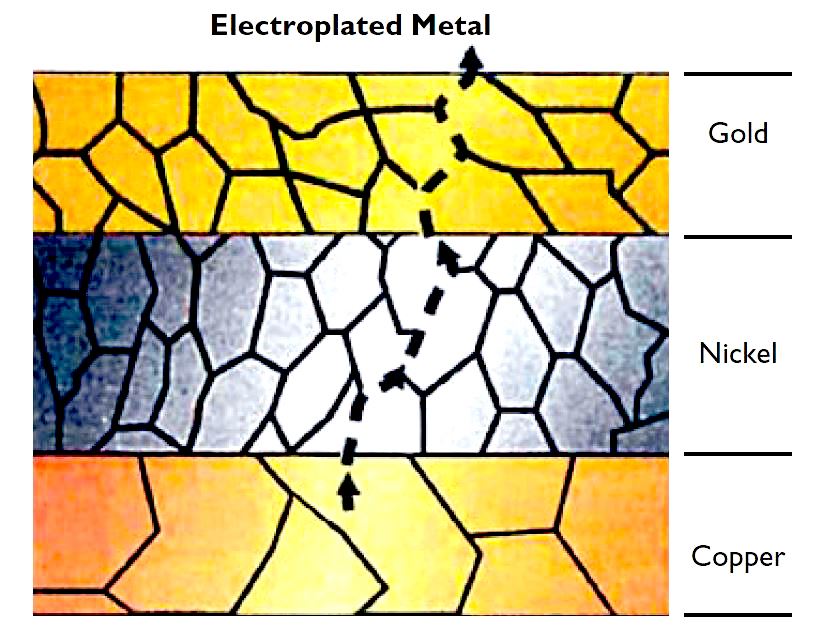
Organic compounds in hard gold electroplated materials (e.g., cobalt organic complex) widen the grain boundaries, which creates shorter, more direct diffusion paths that help base metals diffuse to contact surfaces more easily.
Extreme surface roughness promotes porosity in both clad and electroplated metal materials, but plated surfaces tend to be more vulnerable. Plating more readily accumulates on the peaks and valleys of rough surfaces, which can cause normally acceptable average thicknesses to be poorly distributed. This can leave breaches in the plating surfaces that will go undetected when measuring electroplated coating thicknesses using standard X-ray fluorescence procedures.
Surface roughness also contributes to adhesive and abrasive wear, the basis for which is twofold. First, wear initiates at high surface spots. Second, the uneven distribution of plated gold on rough surfaces makes them more susceptible to excessive wear. For example, a gold-plated connector designed for use in an automobile safety device exhibited unacceptable porosity after just five wear cycles, even though it passed X-ray fluorescence testing, as that process fails to detect poor coating distribution. Although the normal lifecycle of the connector would only be a few wear cycles, routine assembly and diagnostics required an estimated 10 cycles and the specification called for 50 cycles to ensure an acceptable margin of safety.
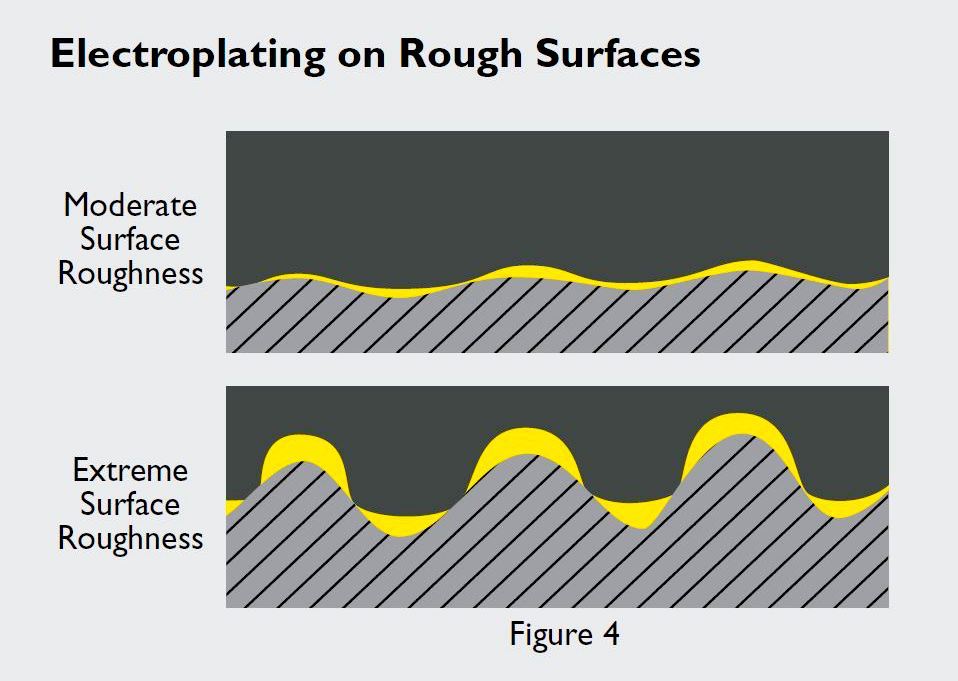
Electroplating more readily accumulates on the peaks and valleys of extremely rough contact surfaces, leaving breaches on the surface that invite galvanic attack.
Surface Coating Testing and Evaluation
Connector manufacturers use two standard tests to monitor their metal coating processes: uncontrolled direct attack testing, which tests for nitric acid and vapor per ASTM B-735, and electrographic controlled corrosion testing per ASTM B-741. These tests also provide an indirect method for predicting service life.
Field exposure can be simulated using Battelle mixed flowing gas environments. Forty-eight hours of this accelerated testing simulates one year of field exposure. The Battelle test creates four classes of environments that represent a range of potential galvanic attack situations. Test classes II and III generate pure corrosion in environments designed to mimic those in which most signal-level connectors are used. Class III testing employs high concentrations of corrosive gases and can generate migrating corrosion that begins to spread a film over an extended area of the contact surface. This phenomenon is called creep and can originate from pores or bare edges where the metal has been stamped. An American Society for Testing and Materials (ASTM) committee is currently developing a specification for class II and III environments in collaboration with a select group of manufacturers that have collected a large amount of data regarding the corrosion resistance of a number of coated metal systems within the Battelle environments.
Reducing Porosity With Gold
Metal coatings less noble than gold, such as palladium, palladium nickel, and palladium silver, are more susceptible to galvanic attack from low levels of ambient chlorine in the atmosphere. This type of galvanic attack can be attenuated by applying a thin coating of gold over these materials, usually 3–5µin of plated gold or 10–20µin of 18-karat clad gold alloys. Numerous lab studies have confirmed that clad gold alloy coatings exhibit much less porosity than thinner plated gold coatings and provide significantly more effective corrosion protection. Moreover, there is no cost penalty to choosing this process because gold alloys are less expensive than the gold used in electroplating processes.
Selecting Contact Surface Coatings
Male connectors typically employ electroplated contacts since the pins are entirely exposed during the mating process and, as such, require environmental and mechanical protection. The cladding process cannot be used for most male connector applications, such as wire pins and stamped blades, but has been successfully employed in stamped and formed fold-over blades.
For female connectors, the cost versus the performance of plated and clad metal materials should be evaluated on the basis of total manufacturing costs. In this situation, clad materials generally combat corrosion at comparable or lower costs because they may be formed after the cladding process, whereas closed-end female connectors are typically preformed, plated, and then finish-formed to avoid cracking the coating, which adds to the total manufacturing cost.
Higher reliability applications require clad metal connectors designed and manufactured to deliver high corrosion resistance. For example, one telecommunications company has used clad metal connectors in its digital switching systems for more than 15 years. During that time, they have had more than five million pounds of pore-free material manufactured for and shipped to them and haven’t returned any of it. This track record, and many others like it, strongly support the use of clad metal connectors in emerging high-reliability communications applications catalyzed by megatrends including Industry 4.0 and 5G.
Conclusion
Galvanic corrosion can be significantly attenuated through thoughtful connector design, materials selection, manufacturing, and handling practices designed to achieve and maintain high-quality contact surface integrity and minimize connector exposure to conditions that promote galvanic attack. When selecting connectors for high-reliability applications, it’s important to keep in mind that a thin gold overcoating can significantly reduce corrosion and that clad gold alloy coatings can offer higher levels of protection without any cost penalty for many female connectors.
Visit Materion Corporation online.
Like this article? Check out our other Connector Basics, contacts, materials, and manufacturing articles, our Consumer Electronics and Telecom/Datacom Market Pages, and our 2020 and 2019 Article Archives.
- Combating Corrosion in High-Reliability Connectors - October 20, 2020
- Connector Contact Materials for Elevated-Temperature Applications - July 28, 2020

