2019 Updates to Medical Standards
Changing medical standards and regulations add complexity to compliance processes required for products and component destined for the medical market.

Medical Standards
Regulations can make designing new products more complicated, more costly, and more time-consuming, adding steps that delay time to market. So why do standards organizations keep setting up these road blocks? Because regulations are designed to make products more compatible, more reliable, and safer. Nowhere is this fact more important than in the world of medical technology, where regulations can be a matter of life or death. Electronic devices can introduce potential risks for electric shock, radiation exposure, chemical contamination, or failure to achieve the intended diagnostic or treatment function. A continuously evolving array of medical standards, requirements, and regulations ensure that electronic products used in clinical settings, in patients’ homes, and inside of people’s bodies remain safe and perform as promised.
Regulatory compliance compels product designers to keep ahead of updates to existing regulations and ensure prepare for compliance with new ones. Regulatory change evolves to ensure that equipment is safe, and new evidence from the field can bring about rapid updates. Care environments are experiencing new levels of interconnection with the electrical grid, wireless networks, and other equipment, and it is essential to maintain safety, reliability, and compatibility.

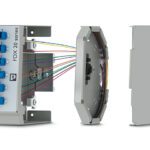
Elipta ECL Series connectors by Smiths Interconnect meet the finger-proofing and IP30 protection requirements of IEC 60601-1, and have been tested per IEC 60529-2004.
Connector suppliers can play a key role in helping designers negotiate the regulatory landscape. Components are designed to meet specific standards or help overall systems meet compliance. “We do not typically work directly with the compliance agencies, but rather with the device designers and end-users to understand their requirements (which includes any agency requirements that need to be met) and design products which can be used in medical devices that must meet these agency requirements,” said Tom Kannally, product specialist and applications engineer at Smiths Interconnect. “Often, the agency requirements are at odds with the size and/or performance requirements specified by the customer; so it’s necessary to either compromise or work closely with the device designer to understand which aspects of the agency requirement are most critical.”
The International Organization for Standardization (ISO) is a non-governmental standards-setting organization that provides common standards for commercial, proprietary, and industrial standards. The efforts of this organization help enable products to be developed and sold across nations, and ensure a level of safety to consumers and end-users. ISO has formed two joint committees with the International Electrotechnical Commission (IEC) to develop standards and terminology for electrical and electronic technologies.
Designers of medical equipment focus on IEC 60601, a series of standards that govern medical devices. A variety of revisions and deviations pertain to specific product categories or countries in which they will be sold. IEC 60601 describes requirements for medical equipment used in the home. It offers safe guidance on matters such as dust and debris exposure, electromagnetic interference, and shock and vibration tests. “Medical devices used at home may be operated by laypersons with no medical training, instead of trained personnel in a controlled hospital environment. Regulatory requirements have been established to provide safety measures specifically for these users,” said Herbert Blum, division components manager at SCHURTER, which offers connectors and other components aimed at high-grade medical equipment.

SCHURTER’s 5707 filtered power entry module with V-Lock cord retention system and hospital-grade cord set.
“The developers of such devices have to observe additional requirements as it relates to safety in addition to specifications about marking (simple operation) and documentation (for example, understandable operating instructions). In particular, the utmost care has to be exercised when it comes to powering the device. Domestic power supply systems, unlike installations in hospitals, are not always reliable or may be insufficiently grounded. A medical device used at home has to compensate for this potential.”
ISO standards, including IEC 60601-1, are updated every five years to keep up with changes to technology, materials, and other considerations. They were last revised on March 31, 2016, and many designers are still in the process of adapting to these latest requirements.
“By far the biggest change that is brought about by the latest edition of IEC 60601-1 is the philosophical shift to a risk management approach, bringing the standard into alignment with the industry’s broad regulatory trends over the past decade,” said Patrick Kinyanjui, an engineer at Fischer Connectors. “The third edition of IEC 60601-1 also revises technical requirements in areas ranging from mechanical hazards, such as crushing or instability to the application of alarm systems. Virtually every one of the standard’s sections incorporates subsections that have special importance for the field of medical connectors.”

Fischer Core Series Plastic disposable circular connectors meet ISO 13484.
Change is the only constant in the regulatory world. Here are some medical standards changes that clinical designers should keep in mind for 2019.
ISO Standards
ISO 13485 is designed to be used by organizations involved in the design, production, installation, and servicing of medical devices, including component manufacturers. It addresses issues such as sterilization, risk management, and inspection and traceability for implantable devices. This standard was last revised on March 31, 2016. This certification prepares devices for international market and is the first step towards compliance with EU regulations.
IEC Standards
The International Electrotechnical Commission (IEC) administers the IECEE standard IEC 60601-1:2020, which guides the safety and performance of medical electronics. This standard is current and far-ranging, encompassing 850 pages of guidance. Product designers should keep these requirements in mind early in the design process. The subsection IEC 60601-1-2, pertaining to EMC requirements in medical devices, went into effect on December 31, 2018. The updated standard, released in late 2020, is expected to remain current until 2025.
MDSAP Certificates
Medical Device Single Audit Program (MDSAP) is designed to help medical device manufacturers to undergo a single audit to meet the requirements of multiple international regulatory bodies. Japan, Australia, Brazil, Canada, the EU, the World Health Organization Prequalification of In Vitro Diagnostics Program, the US Food and Drug Administration (FDA), and the European Union are participants. Other regulatory authorities may participate in the program and its work groups. Device manufacturers also participated in the development of MDSAP’s guidelines. The list of Auditing Organization Availability to Conduct MDSAP Audits is available online. Class II, III, and IV medical devices need a valid MDSAP certificate.
Like this article? Check out our other 2019, Medical Market, and Standards articles.
- News From Hannover Messe 2024 - April 30, 2024
- Where in the World is Amphenol LTW’s Luc Kan? - April 23, 2024
- TE Connectivity’s Sustainability Efforts Pay Off - April 23, 2024