Endoscopes Evolve to Meet Increased Safety Demands
Concerns about cross-contamination have placed scrutiny on minimally invasive medical devices. Electronic components that can withstand enhanced sterilization techniques are essential for reusable equipment. Disposable endoscopes are another possible solution.
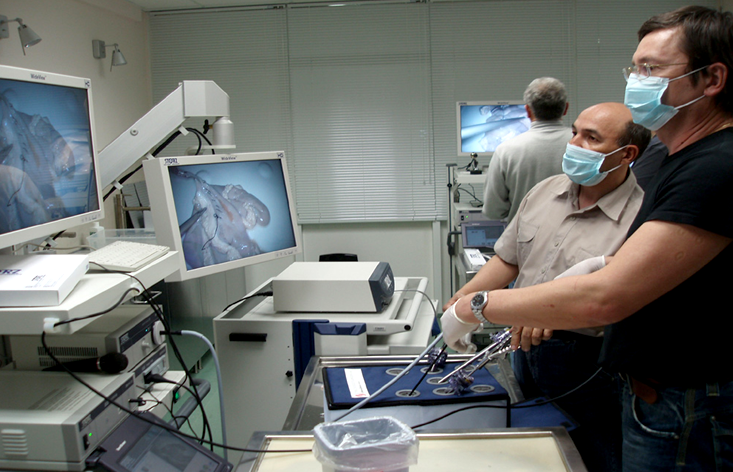
Endoscopy is a minimally invasive procedure in which an illuminated tubular instrument is used to enable physicians to examine a hollow organ, passage, or cavity of the body. Unlike non-invasive diagnostic techniques, endoscopes are inserted directly into the body to perform an internal evaluation of the patient and diagnose or monitor health issues in the esophagus, stomach, colon, ears, nose, throat, heart, and urinary tract.
Bronchoscopes, duodenoscopes, gastrointestinal scopes, and colonoscopes are amongst the most common endoscopes used to visualize areas inside the body and, like most endoscopes, are generally designed to be reusable. Reusable endoscopes typically employ connectors with metal shells, cables with silicone or thermoplastic rubber (TPR) jackets, and liquid rubber silicone (LSR) or TPR overmolding. Endoscopes are hermetically sealed to protect electronics from cleaning, disinfection, and reprocessing procedures, which expose these devices to harsh chemicals and challenging environments, such as autoclave (steam sterilization) or STERIS or STERRAD (low-temperature sterilization) systems. Reusable endoscopes can endure anywhere between 250 and 1,000 cycles, depending on manufacturer warranty, when following reprocessing guidelines.
The U.S. Food and Drug Administration (FDA) continues to remind hospitals and other healthcare providers of the importance of following the strict reprocessing instructions for reusable endoscopes. However, despite this guidance, there have been numerous reported cases of cross-contamination and infectious disease outbreaks associated with reusable medical devices that have led to serious illness or fatalities. The FDA issued warning letters to makers of endoscopes, including Fujifilm, Olympus, and Pentax, instructing them to conduct post-market surveillance studies to better understand how reprocessed devices are still transmitting disease.
Medical endoscopy market experts anticipate that the endoscopy equipment market will exceed $35 billion with a compound annual growth rate (CAGR) of at least 6% and that concerns about the potential for infection will create significantly increased demand for the disposable endoscope market, which is expected to exceed $3 billion with a CAGR of 26% or more by 2026.
Connectivity Products for Reusable Endoscopes
There is a huge installed base for reusable endoscopes and, with rising environmental concerns about medical waste, the market for reusable endoscopes equipped with sterilizable connectors and cable assemblies is expected to remain strong for the foreseeable future. That means device designers must continue to specify connectivity components that are capable of withstanding the most rigorous means of sterilization, including multiple means for reprocessing. Connector suppliers that offer durable metal and plastic connectors suited for endoscopy applications include Amphenol, Carlisle Interconnect Technologies, Fischer Connectors, LEMO, Molex, ODU, Smiths Interconnect, and TE Connectivity.
LEMO offers an extensive portfolio of push-pull metal- and plastic-shell connectors well suited for use in medical and surgical applications. Through its subsidiary Northwire, LEMO is also able to offer vertically integrated connector and cable assemblies equipped with BioCompatic silicon-alternative cable, which offers durability, reliability, and cost-effective pricing; employs materials proven to be non-irritating to even sensitive skin; exhibits robust resistance to chemicals, cuts, and abrasions; is rated to endure more than 500 autoclave, gamma, and sterilization cycles; and is especially well suited for use in endoscopes.

LEMO REDEL SP self-latching, plastic, push-pull connectors have an ergonomic grip, color-coding and keying options, high contact density, and an IP50 seal. They also exhibit high resistance to chemicals, shock, and sterilization processes and are both 100% scoop-proof and compatible with Northwire BioCompatic silicone cable alternative.
Fischer Connectors’ Fischer Core Series features a variety of metal and plastic connectors with silicone and thermoplastic cables. Its circular push-pull connectors have brass shells and IP68 and IP69 or hermetic sealing and can withstand most sterilization methods and more than 10,000 mating cycles, which makes them a good choice for reusable medical devices like endoscopes. Fischer Core Series Plastic connectors offer sealing protection rated up to IP68, can withstand more than 5,000 mating cycles, and are completely sterilizable. Both interconnect solutions are highly configurable, durable, and sterilizable. These connectors are available in a wide range of sizes, configurations, and coding options. Fischer also manufactures silicone and thermoplastic overmolded cable assemblies using their own connectors.

Fischer Connectors’ Fischer Core Series Brass (left) and Plastic (right)
The ODU MINI-SNAP and MEDI-SNAP Series also offer circular, push-pull connectors with metal and plastic shells that are well suited for use in medical applications like endoscopy. MINI-SNAP connectors can withstand more than 5,000 mating cycles and come in IP50 and IP68 moisture-resistant and hermetically sealed versions that may be used for endoscopy applications. MEDI-SNAP connectors are lightweight with versatile mechanical and color-coding options that help prevent mismating and are autoclavable, sterilizable, highly resistant to chemicals, and rated for more than 2,000 mating cycles. In both series, the push-pull connection locks itself into the receptacle and does not release until the outer sleeve is pulled back by the end-user to prevent accidental disconnections caused by pulling on the cable. Both connector series are also available in a wide range of sizes, configurations, and codings and as silicone-overmolded cable assemblies.

ODU MINI-SNAP connectors with metal shells (left) and MEDI-SNAP connectors with plastic shells (right)
Safety Concerns Drive Disposable Endoscopy Market
Field complaints to the FDA are changing the market dynamics affecting the use and reuse of endoscopes. To combat the rising incidence of infections acquired from the use of contaminated endoscopes, healthcare providers are implementing new sterilization processes to prevent inadvertent spread of microbial pathogens from patient to patient. However, the enhanced sterilization processes recommended require specialty cleaning equipment and chemicals for decontamination and increase time and cost required to maintain reusable endoscopes. The FDA has also recommended that healthcare facilities and manufacturers begin transitioning to duodenoscopes with disposable components to reduce risk of patient infection. However, disposable endoscopes create more biohazardous medical waste, which is an environmental threat and may hinder adoption in some markets.
Disposable endoscopes are an emerging technology that integrates components accurate and reliable enough for medical use but inexpensive enough to be suitable for single-patient use, and FDA approvals for these devices are being fast-tracked due to infection outbreaks traced back to reusable models. Major manufacturers of disposable endoscopes include Ambu A/S, Boston Scientific, Flexicare Medical, Hill-Rom Holdings, Karl Storz Imaging, and OBP Medical Corporation. As this market grows, the leading reusable endoscope manufacturers will likely partner with medical device contract manufacturers and connector and cable assembly partners to develop single-patient-use endoscopes. Such collaboration would further drive the development of unique single-patient-use connector and cabling solutions as well.
The FDA recently granted Boston Scientific’s EXALT Model D single-use duodenoscope a Breakthrough Device designation to provide healthcare providers and patients with immediate access to this new disposable technology that can effectively prevent the patient-to-patient pathogen exposure that has been traced back to some reusable endoscopes. This product is the first and only FDA-approved single-use duodenoscope available on the market.

Boston Scientific’s EXALT Model D single-use duodenoscope for gastrointestinal applications is the first and only FDA-cleared single-use duodenoscope on the market.
There are, however, several other types of single-use FDA-approved endoscopes available on the market. AMBU A/S manufactures the aScope 4 Broncho Slim endoscope as a single-use, flexible bronchoscopy solution. With an outer diameter of just 3.8mm, it is ideal for a wide range of procedures. The single-use scope is ergonomic, lightweight, and easy to set up and does not require reprocessing after use. Karl Storz offers several single-use scope products, including the single-use C-MAC FIVE S flexible intubation video endoscope for ear, nose, and throat applications. These single-use solutions feature an ergonomic handle with an integrated LED light source and enable high-quality imaging with image capture and data storage capabilities. Opcom also offers a single-patient-use endoscope that features integrated connectors and complementary metal-oxide-semiconductor (CMOS) sensor technology.
Connectivity Products for Disposable Endoscopes
Onanon offers unique and user-friendly disposable medical connectors for use in single-patient-use medical devices including endoscopes. Some of their disposable connectors feature a magnetic pole that enables easy and intuitive mating and unmating, as well as LED-illuminated receptacles that shine red in the unmated condition and green in the mated condition and vibrate when mated to provide visual, tactile, and audible confirmation of proper connections. Onanon can also integrate wireless connectivity, printed circuitry, or other electronics into custom connectors for medical devices for additional functionality.
Fischer Connectors’ Fischer Core Series also offers disposable push-pull circular connectors for single-use medical applications ranging from catheters and endoscopes to surgical hand tools. The series provides a range of modular, cost-effective, user-friendly solutions that deliver faultless high-reliability performance, are compliant with ISO 13485, and are available in multiple configurations with fully insulated bodies, a quality tactile feel, a snap-lock design, stamped or machined contacts, and color coding for easy identification. They also enable the easy integration of embedded electronics and are available as a pre-cabled turnkey solution for fast and easy integration into any medical device. Fischer Disposable Turnkey Solutions deliver IP65 protection, provide a high degree of electrical insulation, and are compatible with Fischer Core Series receptacles rated at 10,000 mating cycles.
Future Developments
As endoscopy equipment continues to evolve, market leaders are looking for medical connector and cable suppliers and contract manufacturing integrators that can help them develop next-generation endoscope technologies that can reliably endure both the rigors of use and enhanced sterilization techniques designed to better protect patients. These solutions may come in the form of hybrid connector products that offer extended functionality with minimal size and weight, or the market may increasingly turn to a disposable model. The November 2019 MEDICA trade fair in Germany and February 2020 MD&M West conference and exhibition in California both featured several medical device manufacturers showcasing new reusable and disposable technologies for this fast-growing market.
The future of endoscopy is sure to include a variety of both reusable and disposable technologies enabled by new connectivity products proven to go the distance in terms of providing safe, effective care, whether that means withstanding advanced sterilization techniques or contributing to a single-patient-use solution.